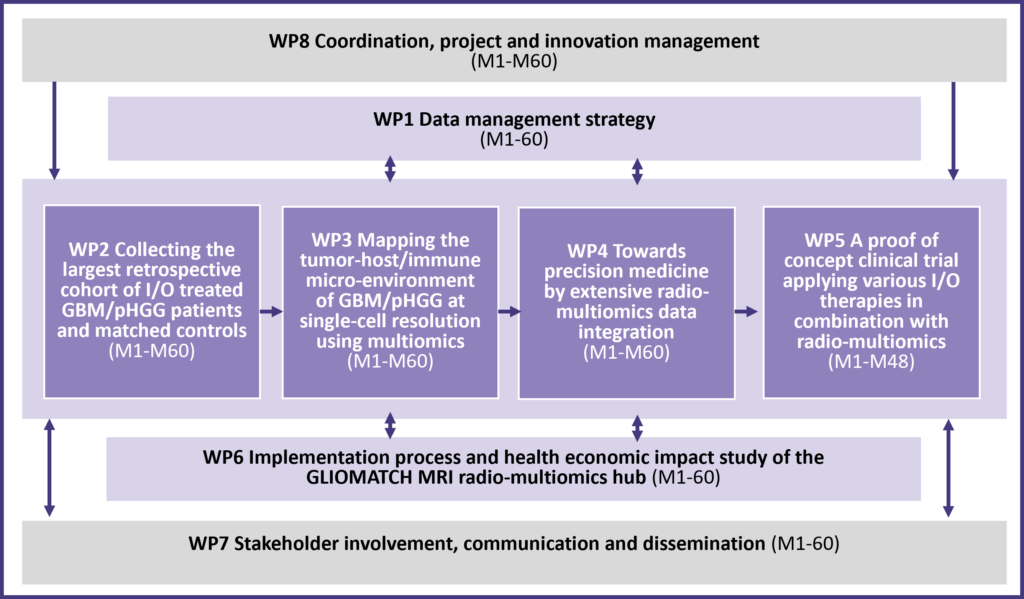
Data management and governance
Lead: KUL
Partners involved: EMC, UM, HSJD, AA, Timelex
Objectives
- Develop a clinical data management plan
- Set up, extend and maintain cloud infrastructure and train consortium stakeholders
- Define legal/ethical framework of data sharing and access policy
- Collection of clinical, pathological and available MRI and NGS data
- Data harmonisation and connectivity with other European data sources, including the UNCAN platform
Collection of retrospective data from cohorts
Lead: KUL
Partners involved: EMC, OUS, FSJD and HSJD, RMC, UDUS, FINCB, UU
Objectives
- Select patient cohorts of GBM and pHGG patients across 8 clinical centres that were treated with immuno-oncology
therapeutics and matched controls - Identify extreme long-term surviving GBM patients across 8 clinical centres
- Define the required clinical information, MRI imaging timepoints and design electronic case report forms used in clinical trials
- Collect formalin-fixed paraffin embedded tissue blocks from all included patients/sampling times
Mapping of the tumour host/immune microenvironment
Lead: KUL
Objectives
- Sample collection, management and tissue microarray generation from collected formalin-fixed paraffin embedded tissue blocks
- Generation of a detailed map of the tumour-host/immune microenvironment in clinical samples using
– spatial transcriptomics
– spatial proteomics
– using spatial (regional) DNA-methylation, chromosomal rearrangement and transcriptomics analysis - Full integration of the three generated spatial maps and cellular network analyses
Patient stratification through Radio-Multiomics data integration
Lead: UM
Partners involved: KUL, AA
Objectives
- Integration of spatial multiomics data in the existing single-cell and bulk omics landscape of GBM/pHGG: towards
a tumour/host-microenvironment patient classifier - Integration of radiology data with clinical outcome
- Enrichment of the model with semantic and clinical features, interpretability and fairness
- Integration of tumour/host cellular interaction networks and clinical outcome
- Integration of tumour/host interaction networks, radiology data and clinical outcome
- Development of an immunotherapy matchmaker algorithm for GBM/pHGG to streamline therapy selection
Implementation of clinical trial for proof-of-concept
Lead: UDUS
Partners involved: KUL, EMC, OUS, FINCB, Timelex
Objectives:
- Design trials, prepare trial documents and obtain legal and ethical approval for the trials
- Set up materials and data flow between trial sites and KUL
- Initiate trial sites and recruit the respective number of patients into the trials (treatment phase)
- Assess systemic and local immunological responsiveness of patients and changes in the tumour microenvironment
- Collect tumour tissue for assessment of composition and immunosuppressiveness of the tumour microenvironment
- Assess safety of treatment
- Assess feasibility of trial design
- Assess survival of patients (follow-up patients clinically)
Social and health economic impact assessment
Lead: UEDIN
Partners involved: CPE, Timelex
Objectives
The main goal of this WP is to conduct an implementation process and health economic impact study of the of the GLIOMATCH MRI Radio-multiomics hub. More explicitly we will:
- Through close collaboration with other WPs, communicate and prepare each participating site for the implementation
study - Appoint and train local implementation study facilitators
- With the help of local facilitators, carry out qualitative investigations to inform development and optimisaion of the therapy selection platform
- Evaluate the health economic impact of the platform in selected sites.
- Carry out qualitative process analyses across early adopter sites to understand mechanisms of action, unintended
consequences and likely generalisability of the platform
Stakeholder involvement, dissemination and exploitation, including communication
Lead: accelCH
Partners involved: KUL, EMC, UM, OUS, HSJD, RMC, UDUS, FINCB, UU, AA, CPE, UEDIN
Objectives
The main objectives of WP7 are to involve stakeholders throughout the project, raise awareness of among patients, policymakers, and clinicians and to disseminate the results achieved adhering to the EC’s open science principles. More specifically, WP7 will:
- Engage and involve all relevant stakeholders such as patient associations, researchers, clinicians, professional associations and healthcare providers throughout the project
- Exchange knowledge with other Cancer Mission-specific coordination actions, notably fostering exchanges with other
proposals funded under this topic - Communicate the urgent need for a better understanding of I/O for GBM patients
- Disseminate project results to the main target groups, including scientific community, healthcare providers and payers,
policymakers, authorities and patients, ensuring open access to the public - Ensure the uptake of commercial and non-commercial project results through exploitation and contribute to enhancing
GLIOMATCH across Europe
Coordination, project and innovation management
Lead: KUL
Partners involved: EMC, UM, OUS, HSJD and FSJD, RMC, UDUS, FINCB, UU, AA, CPE, UEDIN
Objectives
The overall objective of WP8 is to ensure the successful implementation of the project, with the following measures:
- Establish a project-specific governance structure for effective coordination and internal communication
- Facilitate the project organisation and administration according to Horizon Europe rules.
- Prepare periodic, final and progress reports in a timely manner
- Implement a quality assurance plan and risk assessments with execution of contingency measures
- Guarantee of legal and ethics compliance in conjunction with WPs 1 and 6